A New Path Toward Disease Modification
Unlike traditional treatments that only replace lost dopamine, risvodetinib aims to target the cause of neuronal death itself. By restoring the brain’s natural defense systems, c-Abl inhibition may slow or even reverse the progression of Parkinson’s representing a meaningful step toward a true disease-modifying therapy. Risvodetinib blocks both neurodegenerative cell death pathways and neurodegenerative inflammatory pathways, as illustrated in the figure below.
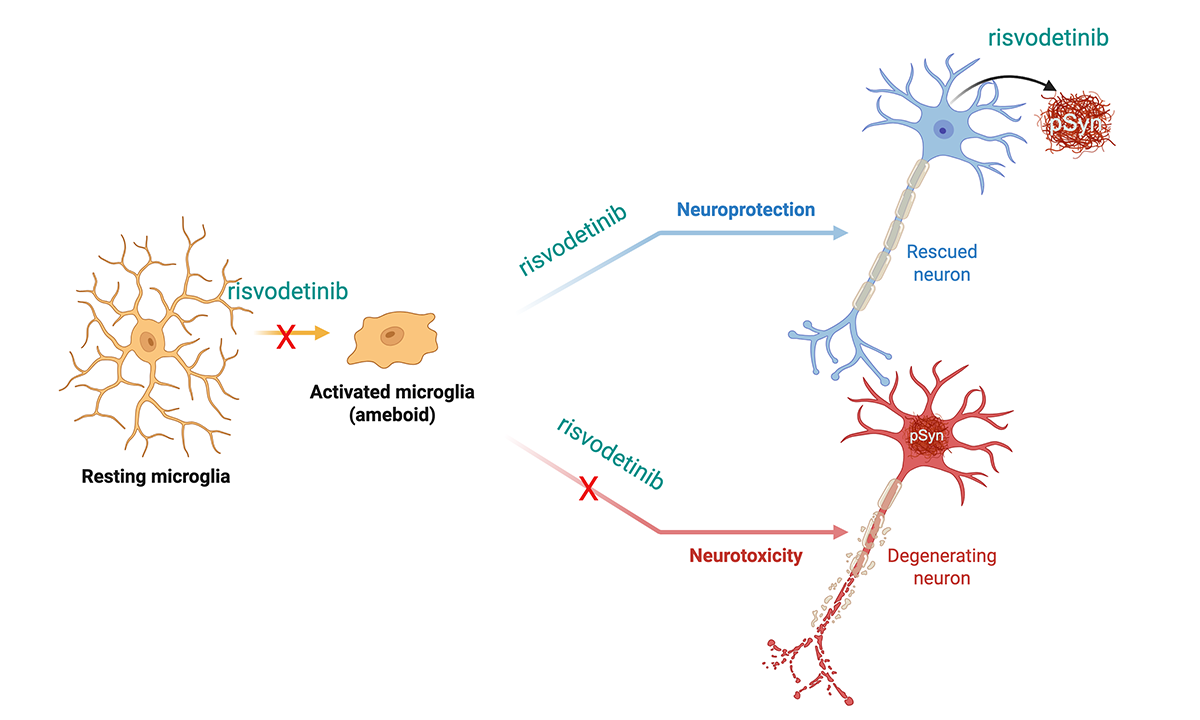
Understanding the Root Cause
In Parkinson’s disease, nerve cells, or neurons, that control movement gradually die because a structural protein alpha-synuclein becomes misfolded.
These misfolded proteins are taken up by neurons and activate a stress-response enzyme called c-Abl. Once triggered, c-Abl modifies alpha-synuclein to create the toxic, disease-causing form. C-Abl also triggers programs of cell death through several mechanisms.
As a result, mitochondria falter, damaged proteins pile up, and a neuron slowly deteriorates leading to the classic motor symptoms of Parkinson’s disease. These processes are represented in the figure below:
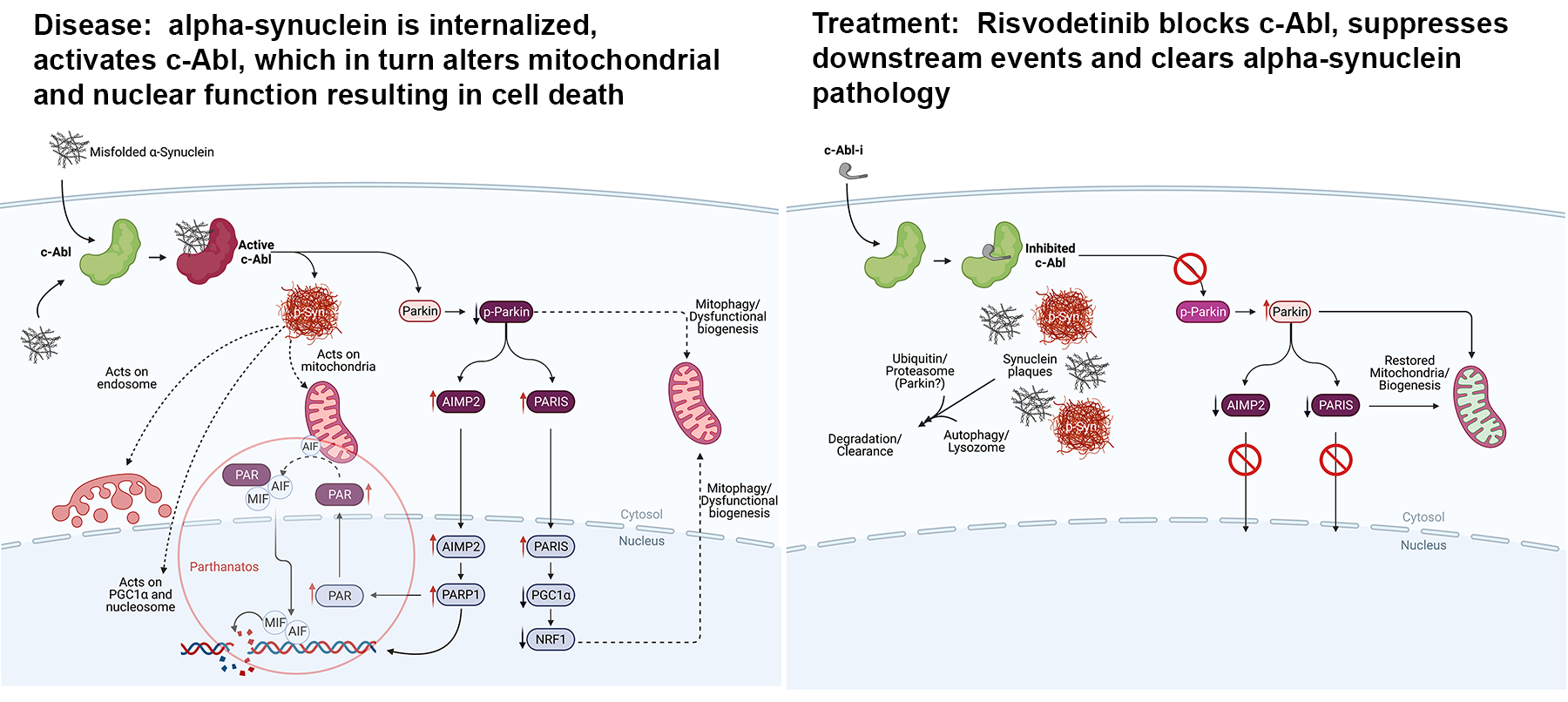
The c-Abl Connection
ABLi’s research has shown that c-Abl acts as a molecular “on-switch” for this destructive process.
When c-Abl is active:
- Toxic alpha-synuclein accumulates in the neuron and can spread to neighboring neurons through tunnels formed between them.
- Parkin, the protein that maintains mitochondrial function and clears toxic proteins, is turned off.
- Cellular repair systems collapse, driving nerve-cell death.
How Risvodetinib Works
Risvodetinib is designed to block c-Abl activity inside the neuron, halting this cycle of destruction.
When c-Abl is inhibited:
- Toxic alpha-synuclein is destroyed within the neuron and then cleared from the cell via endogenous mechanisms like the ubiquitin/proteosome process and autophagy/lysosomal clearance mechanisms.
- Parkin function is restored, allowing the cell to clear toxic alpha-synuclein and restore normal mitochondrial function.
- Nerve cells begin to recover, leading to improvements in both motor and non-motor functions in preclinical studies.